About Us

XL-XDC® Technology Platform:
Pioneering Rapid Development of Drug Conjugates
At the heart of our groundbreaking achievements is the proprietary "Payload + Linker" module library, boasting global intellectual property rights. Through a deep understanding of chemistry and drug delivery, our team has curated an exclusive suite of SuperHydra® Linkers. Meticulously designed for either non-specific or precise site-specific conjugation, these linkers afford us unparalleled control over the Drug Antibody Ratio (DAR). Simultaneously, they elevate conjugate stability and minimize molecular heterogeneity.
Our innovative approach, aptly termed "Choose and Match," strategically pairs antibodies with the optimal "Payload + Linker" modules. This methodology empowers us to create a diverse array of drug conjugate modalities swiftly and effectively, each characterized by optimal properties.
In our portfolio, we have identified a diverse range of both conventional and innovative payloads, each revealing multiple mechanisms of action. These mechanisms span from cytotoxicity and protein degradation to immune stimulation. Going beyond the conventional, we've delved into the realm of dual-payload strategies, enhancing both efficacy and safety margins.
Experience the future of drug conjugate development with Xiling Lab - a convergence of precision, innovation, and transformative potential.
• Selecting the optimal payload for a Drug Conjugate involves a nuanced consideration of various factors. Key determinants include the nature of the targeting disease, mechanism of action (MOA), the specific antibody utilized, potency, stability, immunogenicity, linker compatibility, manufacturability, and other critical parameters. This intricate decision-making process ensures that the chosen payload aligns seamlessly with the desired therapeutic outcome, underlining our commitment to precision and effectiveness in drug development.
• Explore our extensive array of payloads, which encompasses both conventional and novel options, each unlocking diverse mechanisms involving cytotoxicity, protein degradation, and immune stimulation. In addition to these, we take pride in presenting our exclusive payloads, including Eribulin, Lurbinectedin, Trabectedin, Exatecan, and their analogs. This curated selection offers a versatile range of options, meticulously tailored to address various therapeutic needs.
• Leveraging our state-of-the-art facilities, we boast cGMP capabilities essential for the production of payloads and "Payload + Linker" modules. This ensures their meticulous preparation for seamless progression through clinical trials and eventual commercialization.
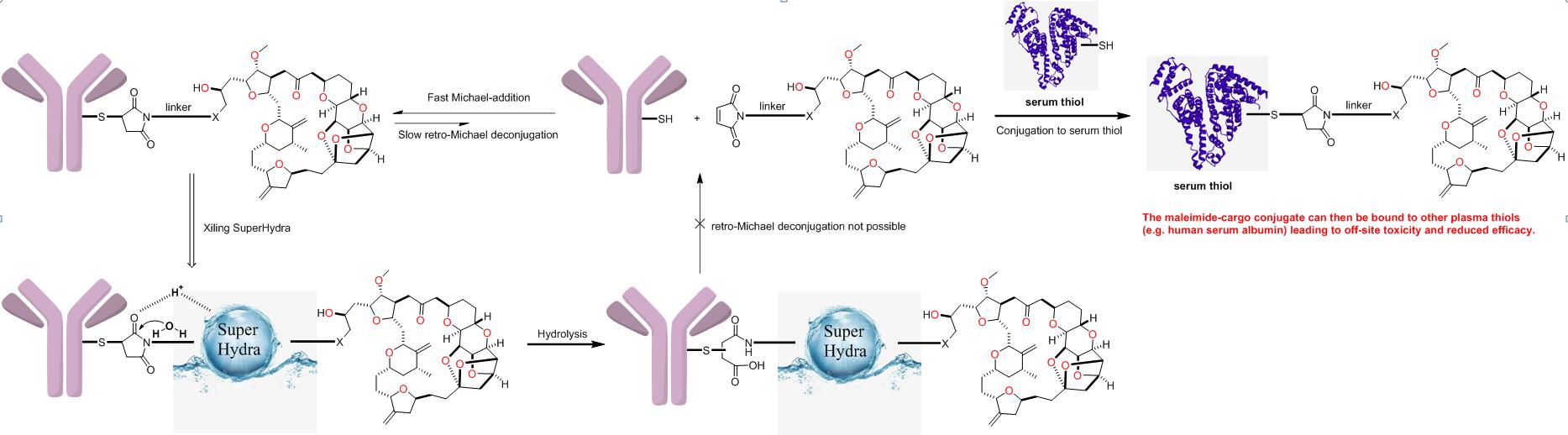
• Nine out fifteen approved ADCs have been constructed through cysteine-maleimide conjugation. This approach has been plagued by retro-Michael de-conjugation, which, in turn, diminishes their efficacy and contributes to undesired off-target toxicity.
• By introducing a SuperHydra® group into the linker, we have successfully initiated a hydrogen-bonding catalyzed ring-opening hydrolysis of maleimide that effectively block retro-Michael de-conjugation. The structure feature is proved to significantly improve conjugate stability during circulation and enhance payload delivery to the target, in turn, allowing higher dosing and better tumor penetration.
• To cater to diverse needs, we have meticulously designed a portfolio from over 100 unique Superhydra® linkers, tailored to match specific antibodies and payloads, offering a comprehensive solution to rapid development of conjugated drugs.
Focusing on the field of oncology, Xiling Lab has developed a pipeline of more than 7 best-in-class and/or first-in-class Drug Conjugates, to address the unmet medical needs of patients worldwide. By leveraging the capabilities of our proprietary "Payload + Linker" module library, we have cultivated a diverse range of innovative modalities, which encompass dual-payload ADCs, Antibody-PROTACs (DACs), and Peptide-Drug Conjugates (PDCs). In parallel, we engage in collaborative partnerships with Biotech and Pharma companies, harnessing the full potential of our XL-XDC technology platform.
SMP-656 is currently in Phase 1 clinical trial (CTR20233290) We envision that SMP-656 holds a great potential to be a best-in-class HER2 ADC, by overcoming primary resistance for approved ADCs or secondary resistance for cytotoxic agents.
.png)
Recruitment:hr@xilinglab.com
Commerce:bd@xilinglab.com

Sweep
Follow The Wechat Official Account